Aniline Hcl Salt. Its conjugate base is the weak base aniline eqC_6H_5NH_2. A salt solution of phenylammonium chloride is formed.
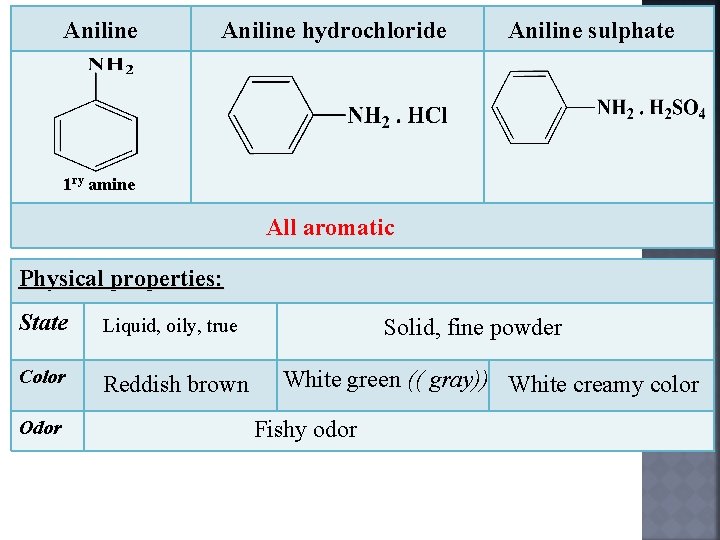
The sulphate forms beautiful white plates. You are just free basing your aniline HCl salt so that it will react with the acetic anhydride. Aniline in solution of its hydrochloride is in form of conjugate acid.
Toxic by ingestion and a skin and eye irritant.
Type in Product Names Product Numbers or CAS Numbers to see suggestions. 245 DegreeC - Melting point. Reacts violently with oxidants. Although aniline is but feebly basic it precipitates zinc aluminium and ferric salts and on warming expels ammonia from its salts.
