Acidic Salt Definition Chemistry. Salts when placed in water will often react with the water to produce H 3 O or OH -. They are formed in the neutralization reaction between a strong acid and a weak base.
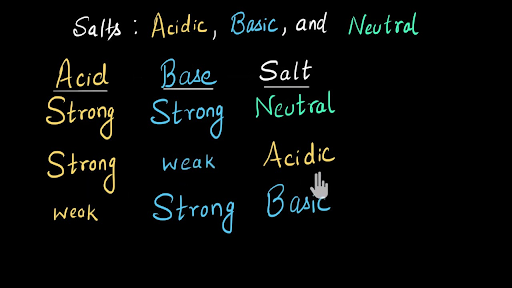
A Normal salt is formed when all the hydrogen ions H of an acid have been replaced by metal ions or by the ammonium ions NH 4 All the salts listed in table 1 are normal salts. A salt is an ionic compound produced by reacting an acid with a base or occurring as a natural mineral. The simple answer is no.
In other words a salt is produced by a neutralization reaction.
When a polybasic acid is not completely neutralized by a base the salt produced will contain replaceable hydrogen atoms. Advertisement Remove all ads. Did this definition by a few chemists become popular. It is always an ionic compound consisting of a positively charged ion called a cation and a negatively charged ion called an anion.
